Corrective actions are executed in response to purchaser problems, unacceptable amounts of products non-conformance, challenges recognized through an inside audit, along with adverse or unstable developments in solution and system monitoring for instance can be discovered by statistical course of action Regulate (copyright).
Up to now Now we have carried out Doc and Education Management and also CAPA and both have everything we want ideal out in the box. Soon after yrs of cumbersome spreadsheets and databases CQ is usually a blessing. It helps make document administration quick and simple… and it’s a enjoyment to utilize. Helen Cary,
Ongoing Advancement: Corrective action is carefully tied towards the principle of continual enhancement. Companies ought to use the lessons learned from the process to improve their units, procedures, and good quality administration procedures constantly.
Our linked suite of answers aids businesses of all sizes maximize merchandise, excellent, security, and supplier as they convey their products from thought to shopper good results. Meet up with the Management Crew
It is possible to email the positioning operator to let them know you were being blocked. Please involve That which you had been executing when this page came up and the Cloudflare Ray ID identified at the bottom of the webpage.
The foremost intent of the CAPA Excellent Management procedure is usually to deal with the foundation triggers of website unique complications and dangers to make sure that there gained’t be a need for either corrective action or preventive action Later on.
Corrective action is not simply a reactive system; It is just a proactive approach to building a culture of excellence and continual advancement in an organization, in the end resulting in bigger achievement in the present aggressive business atmosphere.
Corrective and preventive actions are necessary processes for An effective QMS. They supply a systematic way to deal with weaknesses, which could assist your functions run efficiently whilst keeping away from supplemental fees, delays, and disruption.
An Original correction could possibly be issued in the form of the program patch. After the cause(s) on the bug are identified the corporation will challenge a lasting Answer to the software and employ controls in their progress procedure to stop reoccurrence in the bug.
Investigation teams must determine, get more info Assess, and respond to these deviations and unpredicted events to shield the legal rights, protection, and welfare of contributors and Other individuals along with the integrity on the research information.
The end result can be a application that organizations can leverage to make certain a continuous provide of good quality medicines to individuals.
By submitting this way you concur that we are able to store and method your individual information as per our Privateness Statement. We won't ever sell your own details to any 3rd party.
Assessment the CAPA (along with other procedures if essential) and confirm that there's a mechanism to disseminate applicable CAPA data to These persons straight chargeable for assuring solution high-quality as well as the avoidance of top quality complications.
Utilizing the sampling tables, critique a variety of incomplete failure investigations for probable unresolved solution nonconformances and possible distribution of nonconforming product.
 Alana "Honey Boo Boo" Thompson Then & Now!
Alana "Honey Boo Boo" Thompson Then & Now! Molly Ringwald Then & Now!
Molly Ringwald Then & Now!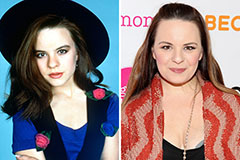 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Joshua Jackson Then & Now!
Joshua Jackson Then & Now! Richard Dean Anderson Then & Now!
Richard Dean Anderson Then & Now!